By Anastasiya Shyshlova, Jim Kong, Calvin Poon, and Certina Ho
Medication incident reporting is becoming a standardized and mandatory practice across many Canadian provinces. Health Canada, the Institute for Safe Medication Practices Canada (ISMP Canada), the Canadian Institute for Health Information (CIHI), and the Canadian Patient Safety Institute (CPSI) collaborate under the Canadian Medication Incident Reporting and Prevention System (CMIRPS) (https://www.ismp-canada.org/cmirps/index.htm) program to capture, analyze and disseminate shared learning from medication incidents.
To determine the understanding of an organization or institution in medication safety, we often look at its safety culture, whether it is “blame and shame”, “reactive”, “calculative”, or “generative” Patient/medication safety culture can be described as shared values, norms, competencies and attitudes towards patient safety among individuals in an organization. Quantitative data alone sets a suboptimal or likely biased foundation for medication incident analysis or evaluation as it has low sensitivity, a lack of a common denominator to quantify the rate of incidents, and is often subjected to misinterpretation when taken out of context. However, qualitative data can provide a detailed narrative and relevant accounts of the incidents. This, in conjunction with quantitative analysis, can provide healthcare professionals with a better understanding and a more complete picture of the lessons learned from the incidents.
At the same time, the analysis of medication incidents can offer an innovative and valuable approach in assessing the patient safety culture of a healthcare institution. Medication Safety Culture Indicator Matrix (MedSCIM) is a novel tool designed by ISMP Canada to assess the quality of qualitative data gathered from medication incidents for a more robust evaluation of the overall medication safety culture in various healthcare settings.
What is MedSCIM?
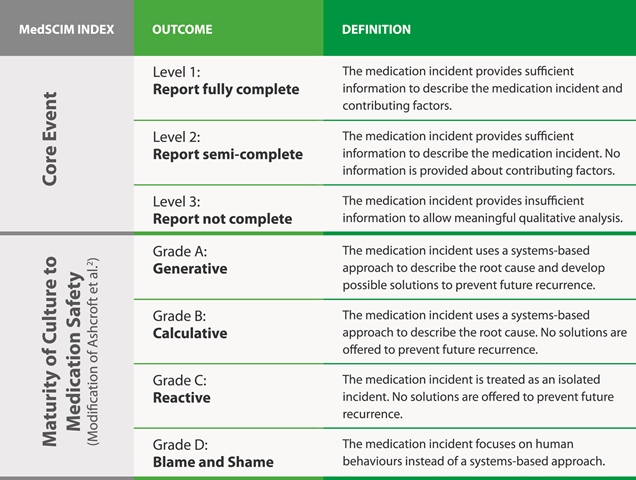
(MedSCIM) was consolidated and validated by obtaining input from an inter-professional patient safety expert panel, consisting of a physician, a registered nurse, and a pharmacy technician. It is comprised of the following two dimensions (Table 1):
- Core Event: This describes the medication incident based on completeness of the documentation submitted by the reporter; it is assigned with a rating of 1 to 3. 4
- Maturity of Culture to Medication Safety: This assesses the reporters’ view and perceived attitude towards medication safety principles and understanding of system-based (rather than individual- or human-based) solutions; it is assigned with a rating of A to D.
This tool can be used as a benchmark for transitioning health organizations from a “blame and shame” work culture with “incomplete reporting” of medication incidents to a “generative” with “fully complete reporting” of medication incidents in regards to maturity of medication safety culture (Table 2).
TABLE 1: MedSCIM dimensions

TABLE 2: Maturity of medication safety culture is defined by colours with red as a negative, yellow as neutral, and green as a positive safety culture
How to Use MedSCIM?
| INCIDENT:
CASE EXAMPLES |
CORE EVENT | MATURITY | PATIENT SAFETY CULTURE LEVEL |
| Coversyl® 2mg was put into the system. However, it was filled as Coversyl® 4mg. The medication incident was discovered at the time of counseling. The patient was given the correct dose. During the time of filling, patient continually interrupted the pharmacist. Patient was upset that it took longer than the promised time to fill the medication. After the incident, a note was made on file. In the future, patient will be told a more accurate wait time. | 1 | A
(Generative) |
Positive |
|
Discussion: The documentation states the error clearly: the incorrect dose of Coversyl® (perindopril) was filled. As well, contributing factors such as constant interruptions were provided in the narrative description. Measures were taken to make sure the error does not happen again, such as documenting a specific note on the patients file, as well, a system change to inform staff to provide more accurate wait times to patients.
|
|||
| Pharmacist noticed that the strength on the hard copy was incorrect. The pharmacy staff member who made the mistake was informed about the error. | 2 | D
(Blame and Shame) |
Negative |
|
Discussion: The documentation stated that the error was an incorrect strength; however contributing factors leading to the error were not mentioned. In terms of maturity, a staff member is singled out and, no proactive measures were taken to prevent this error from reoccurring. As well, system based recommendations to address any potential contributing factors were unaddressed. |
|||
Conclusion
The MedSCIM tool allows for a qualitative analysis and comprehensive understanding of medication incidents to complement traditional quantitative analysis; it provides a framework for assessing the level of medication safety culture in an organization.
Anastasiya Shyshlova is a PharmD Student at the School of Pharmacy, University of Waterloo, and a Medication Safety Analyst at the Institute for Safe Medication Practices Canada (ISMP Canada); Jim Kong is Program Development Manager at ISMP Canada; Calvin Poon is Pharmacy Coordinator at Niagara Health; and Certina Ho is a Project Lead at ISMP Canada.