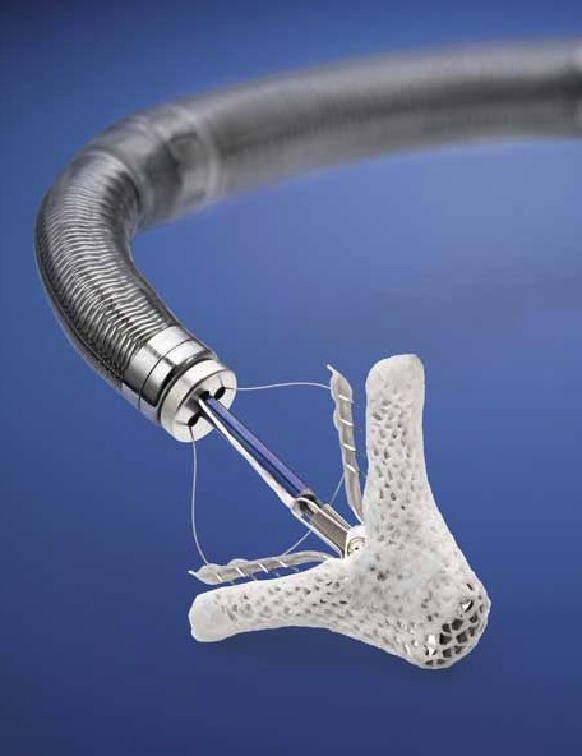
When Health Canada officially approved the revolutionary, catheter-based, MitraClip therapy last April, interventional cardiologists and surgeons at the University of Ottawa Heart Institute knew they had been pioneering an innovative and novel procedure.
Now providing physicians in Canada with a breakthrough treatment option that can significantly improve symptoms, disease progression and quality of life for certain patients with a heart condition called mitral regurgitation (MR), the MitraClip device has been approved for people with degenerative MR who are too high risk for mitral valve surgery based on evaluation by a team of cardiologists and surgeons at the Ottawa Heart Institute.
“This extraordinary achievement in interventional cardiology would not be possible without the exceptional teamwork our highly specialized experts have demonstrated over the last years. Our ability to continuously develop our innovative approach is truly the product of our team’s unique dedication and limitless ambition,” said Dr. Marino Labinaz, Cardiologist and the Director of both the Cardiac Catheterization Laboratory and the Cardiac Fellowship Program at the University of Ottawa Heart Institute.
Degenerative MR is a type of mitral regurgitation caused by an anatomic defect of the mitral valve of the heart. Treatment with the MitraClip device can be effective in reducing the symptoms associated with severe mitral regurgitation, such as shortness of breath and fatigue, which may help people lead a more active lifestyle.
Mitral regurgitation is a common condition, affecting an estimated one in 10 people aged 75 and above. Severe mitral regurgitation can be a debilitating, progressive and life-threatening disease in which a leaky mitral valve causes a backward flow of blood in the heart. The condition can raise the risk of irregular heartbeats, stroke, and heart failure. Open heart mitral valve surgery is the standard-of-care treatment, but many patients are too high risk for an invasive procedure. Medications for the condition are limited to reducing symptoms and do not have the ability to stop the progression of the disease.
MORE: SOUTHLAKE IMPLANTS WORLDS SMALLEST HEART MONITOR IN ADULT PATIENT
“Multiple trials, published reports, and registries of patients treated with the MitraClip device consistently demonstrate a positive safety profile, a reduction in mitral regurgitation, improvements in symptoms, and a reduction in hospitalizations for heart failure,” said Dr. Thierry Mesana, cardiac surgeon and the President and CEO of the University of Ottawa Heart Institute.
Developed by Abbott, the MitraClip repairs the mitral valve without the need for an invasive surgical procedure. The device is delivered to the heart through the femoral vein, a blood vessel in the leg, and once the device is implanted, allows the heart to pump blood more efficiently, thereby relieving symptoms and improving quality of life. Patients undergoing MitraClip treatment typically experience short recovery times and short hospital stays of two to three days.