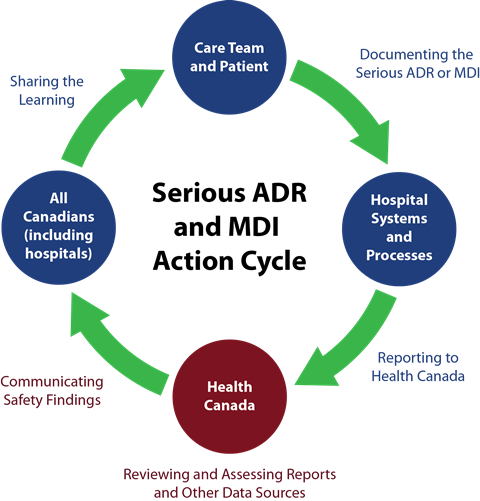
Effective December 16, 2019, reporting of adverse drug reactions (ADRs) and medical device incidents (MDIs) within 30 calendar days of first documentation will be mandatory for all Canadian hospitals. The Protecting Canadians from Unsafe Drugs Act, also known as Vanessa’s Law, is intended to increase drug and medical device safety in Canada by strengthening Health Canada’s ability to collect information and to take quick and appropriate action when a serious health risk is identified.
Four educational modules have been developed to support and raise awareness of mandatory reporting requirements. Available in PowerPoint and PDF formats, these presentations were developed collaboratively by Health Canada, Institute for Safe Medication Practices Canada (ISMP Canada), Health Standards Organization (HSO), and the Canadian Patient Safety Institute. The materials can be used as entire modules, individual slides or selected content for individual learning, or incorporated into presentations for information-sharing. The modules include:
Module 1 – Overview of Vanessa’s Law and Reporting Requirements: explains the purpose of Vanessa’s law, describes the regulations for mandatory reporting of serious ADRs and MDIs by hospitals, and outlines the required data elements for mandatory reporting.
Module 2 – Reporting Processes to Health Canada: describes the expectations for mandatory reporting, provides samples of the forms for reporting and how to submit reports to Health Canada, and includes a number of case studies as examples. The module also includes a Guidance document for reporting and options for voluntary reporting.
Module 3 – Strategies to Promote and Support Mandatory Reporting: identifies potential barriers to serious ADR and MDI reporting, how to facilitate documentation and reporting, and outlines strategies to support implementation. Examples of various reporting systems in place are also included.
Module 4 – Health Canada’s Review and Communication of Safety Findings: summarizes the importance of health product vigilance, the process that will be used to share information on ADR and MDI reporting, and how data will be secured and shared.
All four modules can be accessed on the Canadian Patient Safety Institute website at: https://www.patientsafetyinstitute.ca/en/toolsResources/Vanessas-Law/Pages/default.aspx
Patients for Patient Safety Canada have also created a presentation to help patients and the public understand and promote the reporting of serious adverse drug reactions and medical device incidents. Click here Click here to download the presentation.
For the purposes of mandatory reporting, a serious adverse drug reaction is defined as a noxious and unintended response to a drug that occurs at any dose and that:
- requires in-patient hospitalization or prolongation of existing hospitalization,
- causes congenial malformation,
- results in persistent or significant disability or incapacity,
- is life threatening, or
- results in death.
The mandatory reporting requirements for hospitals apply to therapeutic products, including:
- Pharmaceuticals (prescription and non-prescription drugs),
- Biologic drugs (biotechnology products, fractionated blood products, plasma proteins and vaccines (excluding vaccines administered under a routine immunization program of a province or territory),
- Radiopharmaceutical drugs,
- Disinfectants,
- Medical devices, and
- Drugs for an urgent public health need.
Mandatory reporting does not apply to natural health products, however reporting is encouraged.
A medical device incident is an incident related to a failure of a medical device or a deterioration in its effectiveness, or any inadequacy in its labelling or its directions for use that has led to the death or a serious deterioration in the state of health or a patient, user, or other person, or could do so were it to recur.
All classes of medical devises are included in mandatory reporting by hospitals, including those classified as Class I (lowest risk) to Class IV (highest risk). Examples are:
- Class I – Hospital beds, wheelchairs, leg prostheses,
- Class II – infusion sets, syringes, tracheostomy tubes, urethral catheters,
- Class III – infusion pumps anesthesia gas machines, intrauterine devices, and
- Class IV – pacemakers, defibrillators, breast implants, bone grafts.
More information on mandatory reporting is available on the Health Canada website.
This article was contributed by the Canadian Patient Safety Institute.