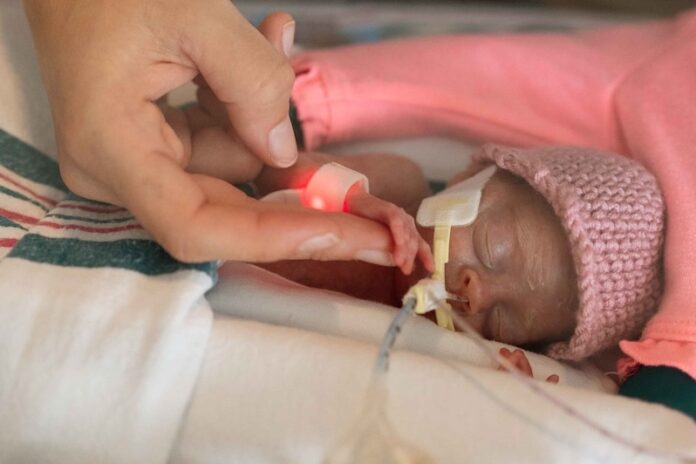
At two weeks old, Emerson Cogan was already a pioneer.
Born February 20, 2023, at 23 weeks gestation and weighing 515 grams, Emmy was the first baby to receive cell therapy in a world-first Phase 1 clinical trial.
The goal of this therapy is to prevent or lessen the effects of a chronic lung disease called bronchopulmonary dysplasia (BPD). Around 1,000 premature babies in Canada develop BPD every year, and there is no cure.
“Treating our first patient in this trial was a dream come true,” said study lead Dr. Bernard Thébaud, a neonatologist and senior scientist at The Ottawa Hospital and CHEO and professor at the University of Ottawa. “Our team had been working toward this moment for 18 years, ever since we discovered that stem cells from the umbilical cord could protect and heal newborn lungs in the lab.”
Joining the study: A careful decision
Emmy arrived much earlier than expected, and the first weeks of her life were challenging. She needed a ventilator and extra oxygen to help her breathe, which put her at risk of BPD.
The research team told Emmy’s parents Alicia Racine and Mike Cogan that she was a good candidate for the trial. After consulting with trusted friends, family and healthcare providers, Alicia and Mike decided to enroll her.

“Being a preemie, Emmy’s going to have some health issues. And anything that could help, we wanted to give her that extra shot,” said Mike.
“The few days after she got the treatment she improved really well, so that made us feel like we had made the right decision,” remembers Alicia. “We don’t know what she would be like without it. But she’s pretty awesome right now, and we don’t think it had any negative effects.”
Trial is just the beginning
The goal of this Phase 1 trial is to determine whether the cell therapy is safe and feasible for premature babies, and to find the best dose for future trials.
“As much as it was just the beginning stages of a trial and the dosage was fairly low, it was still a very positive step for her and for future babies in her position. We were all for it,” said Mike.
After four months in the neonatal intensive care unit (NICU), Emmy finally got to go home on June 30, 2023.
“Emmy does have BPD from being on a ventilator,” says Alicia. “The winter will be the true test of the BPD. We’ll see what kind of load that’s going to put on us, trying to keep her and ourselves healthy and not ending up back in the hospital.”
“We’re seeing promising signs”
“Treating our first patient in this trial was a dream come true,” said study lead Dr. Bernard Thébaud, a neonatologist and senior scientist at The Ottawa Hospital and CHEO and professor at the University of Ottawa.This trial has completed recruitment of nine very premature babies.
They were recruited from NICUs at The Ottawa Hospital and Sunnybrook Health Sciences Centre. The Ottawa participants will have regular follow-up visits at CHEO as part of the trial.
“All of the babies in the trial are doing well so far,” said Dr. Thébaud. “We’re seeing promising signs that the cells are not only safe, but may also be improving their outcomes. But we won’t know that for sure until we can do a larger trial. This safety trial is a critical step towards a potential breakthrough therapy that could help premature babies in Canada and around the world.”
This trial is funded by the Stem Cell Network with in-kind matching funds from MDTB Cells GmbH. Dr. Thébaud’s research is also possible because of funding from the Ontario Institute for Regenerative Medicine, the Canadian Institutes of Health Research, The Ottawa Hospital Foundation and the CHEO Foundation. His research is also enabled by Core Resources at The Ottawa Hospital such as the Ottawa Methods Centre, the Biotherapeutics Manufacturing Centre, and the BLUEPRINT Translational Research Group.

