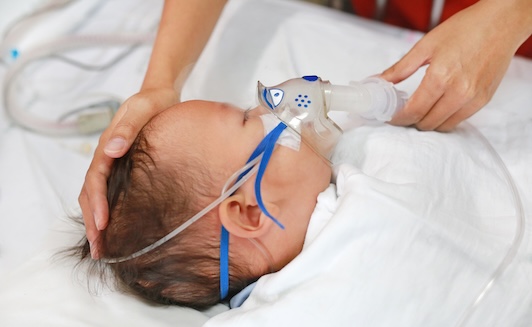
Ottawa mother Jessica Cohn still vividly remembers crumpling to the hospital floor when a team of doctors told her that her one-month-old may need to be intubated. Her healthy full-term newborn son Eitan had been diagnosed with RSV, a highly contagious virus that can be deadly for infants. While infants with some medical conditions are at higher risk, most infants diagnosed with severe RSV disease each year are healthy infants like Eitan. Eitan did recover, but for parents like Jessica, the emotional distress leaves a lasting mark.
RSV is very contagious and causes seasonal epidemics, like the flu, with cases peaking in the fall and winter months. In addition to the psychology trauma RSV inflicts on families, the disease also puts strain on our pediatric units. A significant portion of RSV-related hospitalizations are admitted to the ICU and over 60% of those admissions are for children under 6 months old. The economic impact of these RSV-associated hospitalizations is high, with an estimated cost of $9,000 per case in Ontario.
Until this year, protections against RSV for healthy infants in Canada were limited to basic hygiene and keeping children away from people with colds. Now, Health Canada has approved two new products that offer protection against RSV.
• Nirsevimab is a monoclonal antibody which can be given to newborns and infants in their first RSV season and to high-risk children up to 24 months of age in their second RSV season, and
• RSVpreF a maternal RSV vaccine that can be given to pregnant people who are between 32-36 weeks pregnant. The vaccine protects babies 0 to 6 months through protection from their mothers.
These tools have incredible promise for reducing the impact of RSV in Ontario and across Canada, but several barriers stand in the way including low awareness, and in some provinces, cost.
In Ontario, both Nirsevimab and RSVpreF are available free of charge to eligible parents and infants. This is not the case in most provinces where parents must pay out of pocket for RSVpreF. Nirsevimab is funded through publicly funded programs, but is not available in all provinces. These restrictions limit options for parents. In most cases however, it is a lack of awareness preventing both doctors and patients from having informed discussions about the available options.
We need policymakers to act now to bridge knowledge gaps for parents and healthcare providers and to equip healthcare providers with the communication tools to support informed-shared decision making. We need clear and consistent communication about the availability of RSV protections in Ontario and how parents can access them. But most importantly, we need to reach parents now to inform them about their options. We need both a broad and tailored approach to communicating with parents, making sure that information is accessible and relevant to all pregnant people in all communities.
When Jessica Cohn was pregnant with her third child, her first since Eitan, she could only dream of a vaccine that could protect her child from this ex
tremely common disease. We cannot afford to squander the incredible opportunity we have to save another parent the trauma of seeing their tiny child in a hospital bed, struggling to breathe. We have the tools to prevent RSV this season, but we need act now so parents can make the right choice for them and their families.
For more details on the Federation of Medical Women and the recently published Maternal RSV White Paper, please visit fmwc.ca.