Scientists at the Montreal Neurological Institute and Hospital have used a powerful tool to better understand the progression of late-onset Alzheimer’s disease (LOAD), identifying its first physiological signs.
Led by Dr. Alan Evans, a professor of neurology, neurosurgery and biomedical engineering at the Neuro, the researchers analyzed more than 7,700 brain images from 1,171 people in various stages of Alzheimer’s progression using a variety of techniques including magnetic resonance imaging (MRI) and positron emission tomography (PET). Blood and cerebrospinal fluid were also analyzed, as well as the subjects’ level of cognition.
The researchers found that, contrary to previous understanding, the first physiological sign of Alzheimer’s disease is a decrease in blood flow in the brain. An increase in amyloid protein was considered to be the first detectable sign of Alzheimer’s. While amyloid certainly plays a role, this study finds that changes in blood flow are the earliest known warning sign of Alzheimer’s. The study also found that changes in cognition begin earlier in the progression than previously believed.
Late-onset Alzheimer’s disease is an incredibly complex disease but an equally important one to understand. It is not caused by any one neurological mechanism but is a result of several associated mechanisms in the brain. LOAD is the most common cause of human dementia and an understanding of the interactions between its various mechanisms is important to develop treatments.
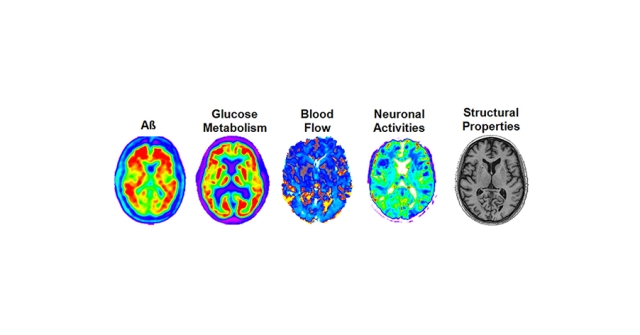
Previous research on the many mechanisms that make up LOAD has been limited in scope and did not provide a complete picture of this complex disease. This study, published in the journal Nature Communications on June 21, factored in the pattern of amyloid concentration, glucose metabolism, cerebral blood flow, functional activity and brain atrophy in 78 regions of the brain, covering all grey matter.
“The lack of an integrative understanding of LOAD pathology, its multifactorial mechanisms, is a crucial obstacle for the development of effective, disease-modifying therapeutic agents,” says Yasser Iturria Medina, a post-doctoral fellow at the MNI and the paper’s first author.
The trajectory of each biological factor was recorded using data from each patient taken over a 30-year period. This process was then repeated 500 times to improve robustness of estimations and stability of the results.
Compiling and analyzing the data took thousands of compute hours to complete, and could not have been possible without sophisticated software and terabytes of hard drive space. Such a data-driven approach to neurology is becoming increasingly important, according to Evans.
“We have many ways to capture data about the brain, but what are you supposed to do with all this data?” he says. “Increasingly, neurology is limited by the ability to take all this information together and make sense of it. This creates complex mathematical and statistical challenges but that’s where the future of clinical research in the brain lies.”
This research also underlines the importance of data sharing across institutions, known as the Open Science model. Patient data for the study came from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a partnership of more than 30 institutions across Canada and the United States. The knowledge that this study has added to our understanding of LOAD would still be undiscovered had it not been for data sharing. Evans points out that his is just one of hundreds of scientific papers to come from the ADNI dataset.
“That by itself is justification for ADNI and data sharing,” he says. “What goes around comes around. We benefit from the data put in by others, and we contribute our own data.”
While this study is one of the most thorough ever published on the subject of Alzheimer’s disease progression, Evans says he would like to go further, to not only record but determine the causes of each mechanism, which could be the key to unlocking better treatments. It is something that is limited only by how much computer power Big Data can provide.
“This is a computational, mathematical challenge that goes beyond anything we’ve done so far,” says Evans. “Our goal is to go to a high-level, causal modeling of the interactions amongst all of the factors of disease, but you need huge computational power to do that. It’s our job to be ready with the software, the algorithms, and the data while we wait for the hardware to appear.”
“We still need more data-driven integrative studies, capable of considering all possible biological factors involved, as well as of clarifying the direct interactions among these factors,” says Medina. “Without that, we cannot dream of effective treatments. We would continue to work in the dark.”